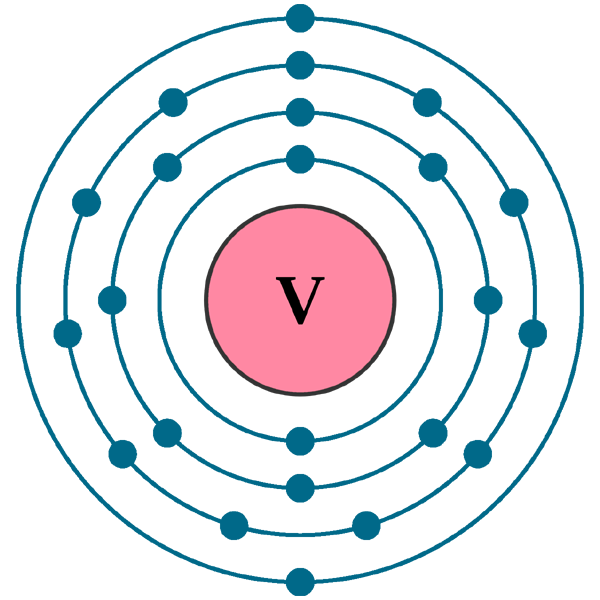
Electron Configuration of Vanadium: A Detailed Explanation
In this article, we will use diagrams, examples, and principles to illustrate the electron configuration of vanadium and its ions.
Is the structure of an atom’s electron cloud a mystery to you? How do they decide how a substance will react chemically? When a substance generates ions, what happens to it? This blog article was written for everyone who shares similar concerns. In this article, we will use diagrams, examples, and principles to illustrate the electron configuration of vanadium and its ions.
The arrangement of electrons in an atom’s orbitals is known as its electron configuration. The most common places for electrons to hang around are called “orbitals,” and they’re located everywhere around the nucleus. Two electrons with opposing spins may coexist in a same orbital. Orbitals are organised into shells with increasing energies from 1 to 7. Subshells, also known as sublevels, may be found inside each shell and are designated by the letters s, p, d, and f. Two electrons fit in the s subshell, six fit in the p subshell, ten fit in the d subshell, and fourteen fit in the f subshell.
The number of electrons in each orbital of an element may be calculated from its electron configuration. Understanding the valence electrons, oxidation states, and bonding capacities of an element requires knowledge of its electron configuration. The outermost shell of an atom contains the valence electrons that participate in chemical processes.
When producing ions or compounds, atoms acquire or lose electrons, and these changes are quantified by a number called the oxidation state of the atom. The capacity of an atom to share or transfer electrons with other atoms in order to form bonds is referred to as its bonding ability.
The chemical symbol for vanadium is V and its atomic number is 23. It is a hard, silvery-gray metal that sits in the periodic table’s group for transition metals. It may operate as a catalyst in chemical processes, can be found in batteries and alloys, and can even be found in living organisms as a trace element.
The Electron Configuration of Vanadium
Vanadium’s electron configuration may be written by following the guidelines for how to fill orbitals. These regulations are:
- The aufbau principle: According to the aufbau principle, orbitals must be filled in order, starting at the ground state and progressing to the greatest energy level. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p are the appropriate positions in which to fill.
- The Pauli exclusion principle: According to this law, each orbital may host a maximum of two electrons with antiparallel spins. Electrons behave like small magnets due to a feature called “spin.” To illustrate electron spin, we utilise arrows pointing upwards and downwards.
- Hund’s rule: According to Hund’s rule, we must first put one electron in each of the empty orbitals of equal energy (such as p, d, or f orbitals) before we can couple them up. This maximises the abundance of electrons with parallel spins that are unpaired.
Following these guidelines, we may express vanadium’s electron configuration in both the complete notation and the noble gas notation.
- The entire notation displays all of an element’s orbitals and their associated electrons. Vanadium’s atomic structure consists of 23 electrons, all of which must be placed in the lowest energy level (1s). Vanadium’s complete chemical symbol is
- The noble gas notation is a simplification of the complete notation in which the core electrons (the innermost electrons that are not engaged in chemical processes) are represented by the symbol of the preceding noble gas in brackets. For vanadium, the first 18 electrons are represented by [Ar], while the last 5 electrons are valence electrons. Vanadium’s designation as a noble gas is:
Electron Configuration of Vanadium may be represented graphically by a schematic of the element’s orbitals and electrons. The electrons are shown as arrows, and the orbitals as boxes or lines. Vanadium’s schematic looks like this:
4s ____
↑↓
3p ____ ____ ____
↑↓ ↑↓ ↑↓
3s ____
↑↓
2p ____ ____ ____
↑↓ ↑↓ ↑↓
2s ____
↑↓
1s ____
↑↓
3d ____ ____ ____ ____ ____
↑ ↑ ↑To some extent, vanadium’s valence electrons, oxidation states, and bonding capacities are determined by its electron configuration. Two of vanadium’s valence electrons are in the 4s shell, while the other three are in the 3d shell. Changing the number of electrons in vanadium’s outermost shell or subshell results in its oxidation state changing from -1 to +5. Its most prevalent oxidation numbers are 2, 3, and 5. Vanadium may share or transfer electrons with other elements to create a variety of bonds, including metallic, covalent, and ionic connections.
The Electron Configuration of Vanadium Ions
By giving up or accepting electrons from its outermost shell or subshell, vanadium may produce positive or negative ions. Vanadium loses electrons from its 4s orbital before it can form a positive ion (cation), and subsequently from its 3d orbital. Vanadium acquires electrons in its 3d orbital and subsequently its 4s orbital when it creates negative ions (anions). Most vanadium ions have the following electron configurations.
- V2+: Ion with charge of +2, obtained by stripping two electrons from an otherwise neutral atom; symbol V2+. V2+ has the following electron configuration:
The diagram for V2+ is:
4s ____
3p ____ ____ ____
↑↓ ↑↓ ↑↓
3s ____
↑↓
2p ____ ____ ____
↑↓ ↑↓ ↑↓
2s ____
↑↓
1s ____
↑↓
3d ____ ____ ____ ____ ____
↑ ↑ ↑
- V3+: Ion with a charge of +3, obtained by stripping three electrons from an otherwise neutral atom; symbol V3+. V3+ has the following electron configuration:
The diagram for V3+ is:
-
4s ____ 3p ____ ____ ____ ↑↓ ↑↓ ↑↓ 3s ____ ↑↓ 2p ____ ____ ____ ↑↓ ↑↓ ↑↓ 2s ____ ↑↓ 1s ____ ↑↓ 3d ____ ____ ____ ____ ____ ↑ ↑V4+: The loss of four electrons from a neutral atom results in the V4+ ion, which has a charge of +4. Its electron configuration, V4+, is as follows:
The diagram for V4+ is:
4s ____
3p ____ ____ ____
↑↓ ↑↓ ↑↓
3s ____
↑↓
2p ____ ____ ____
↑↓ ↑↓ ↑↓
2s ____
↑↓
1s ____
↑↓
3d ____ ____ ____ ____ ____
↑
V5+: If an ion has a charge of +5, it signifies that it has lost five electrons from its neutral atom to become positively charged (V5+). This is the electron configuration of V5+:
The diagram for V5+ is:
4s ____
3p ____ ____ ____
↑↓ ↑↓ ↑↓
3s ____
↑↓
2p ____ ____ ____
↑↓ ↑↓ ↑↓
2s ____
↑↓
1s ____
↑↓
3d ____ ____ ____ ____ ____
Each vanadium ion has a unique electron configuration that determines its stability, reactivity, and colour. Ion stability and reactivity tend to degrade with increasing electron loss or gain. This is because the equilibrium between the nuclear attraction and the electrical repulsion among the electrons is disrupted whenever an atom loses or gains an electron.
Because it lacks any remaining valence electrons and has a very high positive charge, V5+ is the most reactive and unstable vanadium ion. The energy gap between the d orbitals, which in turn affects the colour, is likewise affected by the ion’s positive charge. When the energy difference between the electron and the ion is considerable, the ion absorbs more visible light, giving the ion a more vibrant colour.
Conclusion
Using diagrams, examples, and principles, this essay explains in depth the electron configuration of vanadium and its ions. The electron configuration of vanadium has been written and deciphered utilising our knowledge of the aufbau principle, the Pauli exclusion principle, and Hund’s rule. We have also discovered that vanadium’s valence electrons, oxidation states, and bonding capacities are all impacted by the element’s electron configuration. What’s more, we know that vanadium may shed or pick up electrons from its outermost shell or subshell to generate positive or negative ions.
We appreciate you taking the time to read this page, and we hope you found it interesting and informative concerning vanadium and Electron Configuration of Vanadium. Feel free to leave a remark below with your thoughts, questions, or recommendations. A heartfelt “thank you” for reading this far.
You Can Also Read Here Yimusanfendi: A Leader in Big Data Analytics and Solutions
